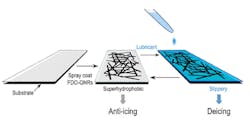
Rice University scientists have advanced their graphene-based de-icer to serve a dual purpose. The new material still melts ice from wings and wires when conditions get too cold. But if the air is above 7 degrees F, ice won’t form at all.
The Rice lab of chemist James Tour gave its de-icer superhydrophobic (water-repelling) capabilities that passively prevent water from freezing above 7 degrees. The tough film that forms when the de-icer is sprayed on a surface is made of atom-thin graphene nanoribbons that are conductive, so the material can also be heated with electricity to melt ice and snow in colder conditions.
This material can be spray-coated, making it suitable for large applications, according to the researchers. The study was published in the American Chemical Society journal ACS Applied Materials and Interfaces recently.
Tour; lead authors Tuo Wang, a Rice graduate student; and Yonghao Zheng, a Rice postdoctoral researcher, and their colleagues tested the film on glass and plastic.
Materials are superhydrophobic if they have a water-contact angle larger than 150 degrees. The term refers to the angle at which the surface of the water meets the surface of the material. The greater the beading, the higher the angle. An angle of 0 degrees is basically a puddle, while a maximum angle of 180 degrees defines a sphere just touching the surface.
The Rice films use graphene nanoribbons modified with a fluorine compound to enhance hydrophobicity. They found that nanoribbons modified with longer perfluorinated chains resulted in films with a higher contact angle, suggesting that the films are tunable for particular conditions, Tour said.
Warming test surfaces to room temperature and cooling again had no effect on the film’s properties, he said.
The researchers discovered that below 7 degrees, water would condense within the structure’s pores, causing the surface to lose both superhydrophobic and ice-phobic properties. At that point, applying at least 12 volts of electricity warmed it enough to retain repellant properties.
Applying 40 volts to the film brought it to room temperature, even if the ambient temperature was –25 degrees F. Ice allowed to form at that temperature melted after 90 seconds of resistive heating.
The researchers found that while effective, the de-icing mode did not remove water completely. Some remained trapped in pores between linked nanoribbon bundles. Adding a lubricant with a low melting point (–61 degrees F) to the film made the surface slippery, sped de-icing, and saved energy.
Co-authors of the paper are Rice alumnus Abdul-Rahman Raji, now a postdoctoral researcher at the University of Cambridge; and Rice graduate students Yilun Li and William Sikkema. Tour is the T T and W F Chao Chair in Chemistry as well as a professor of computer science and of materials science and nanoengineering.
The Air Force Office of Scientific Research supported the research.
Read the abstract at http://pubs.acs.org/doi/abs/10.1021/acsami.6b03060